4. Error Messages and T
4.1 Error Messages
4.2 T
5. Maintenance and
5.1 Maintenance
T
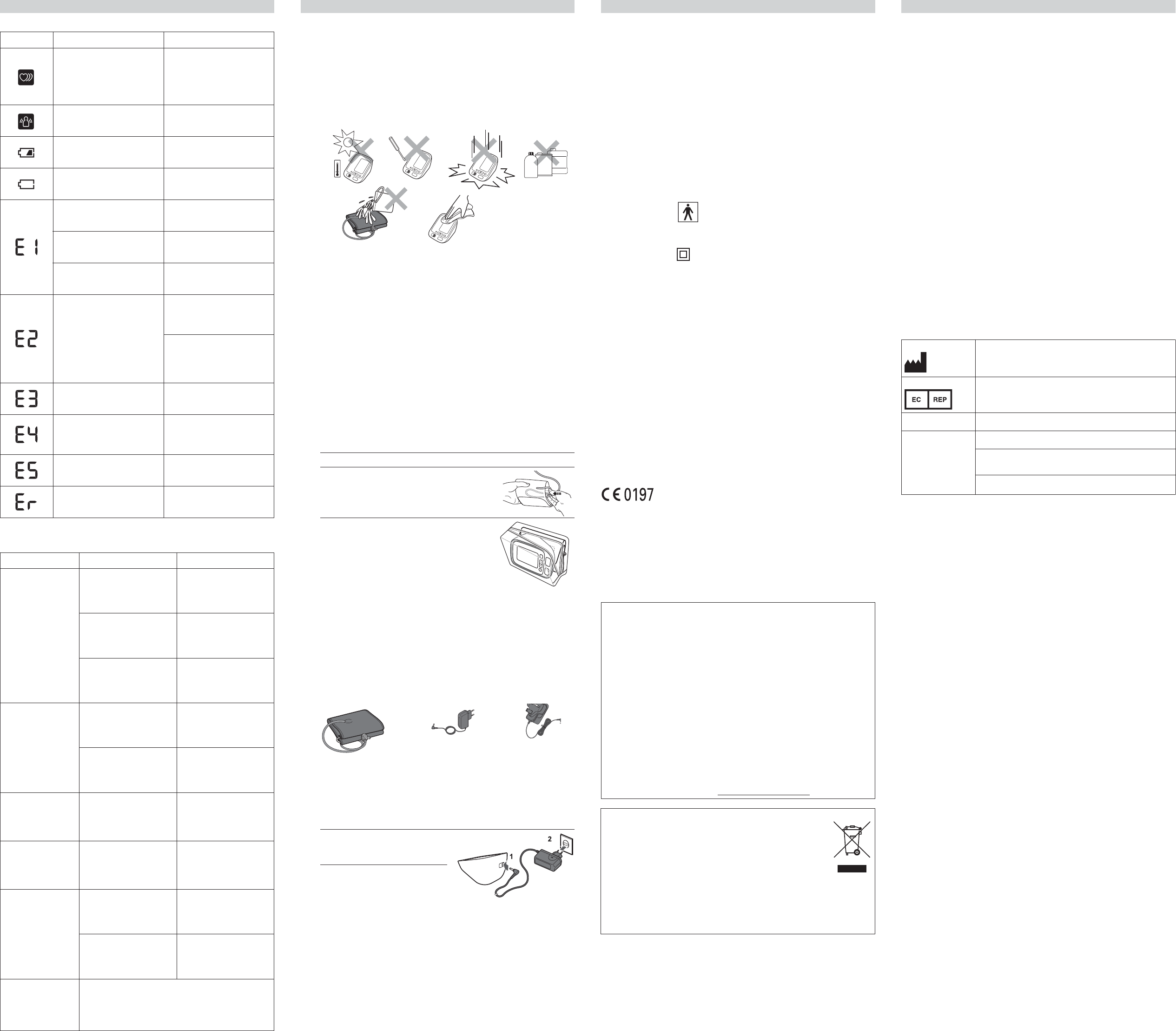
• S
locat
• Do not u
• Do not wa
them in wat
• Do not u
device.
• Use a soft and
neutral
• Changes o
will void
repair the dev
OMRON retail outlet o
Calibration and Servi
• The accur
careful
• It is gene
every
Plea
distributo
5.2 Stor
Keep the device in it
1. Unplug th
2. Gently
Note: Do not bend or crease the air tube exce
3. Place th
storag
Do not store the device in the
•If the dev
•Locations exp
humidity
vapours.
•Locations exp
where it will be at
5.3 Optional Medi
(within the
Using the Optional AC Adapter
1. Insert
into the A
the rear side of
2. Plug the
electric
T
first and then remove the AC adapter plug f
6. S
Notes:
• These specific
• In the clinical v
determination of diastolic blood
• This device has
• This device
Directive).
• This blood pressure monitor is designed according to t
EN1060, Non-invasive sphygmom
Part 3: Supplementary requirem
measuring syst
• This OMRON product is produced under the strict qualit
HEAL
monitors, which is the Pressure Sensor
7. W
Thank you for buying an OMRON product. This product is constructed of high
quality materials and great care has been t
to give you every satisfaction, provided that it is properly operated
as described in the instruction manual.
This product is
purchase. The pr
guaranteed by OMRON. During this period of guarantee OMRON will, without
charge for labour
parts.
The guarantee does
a. Tr
b. Costs for repairs and /
persons.
c. Periodic check
d. Failure or w
itself, unless explicitl
e. Costs arising due t
f. Damages of any kind including personal caused accident
g. Calibration service is not
h. Optional parts have a one (1) year warranty f
include, but are not limited to the foll
Should guarantee ser
was purchased
the product packaging / lit
If you have difficulties in
information.
www
Repair or replacement under the guarantee does not give
renewal of the guarantee period.
The guarantee will be granted only if the complete product is returned together with
the original invoice /
Made in Vietnam
Error Display Cause Solution
Irregular heartbeats are
detected.
Remove the
minutes and then take another
measurement. Repeat the steps
in section 3.3. If this error
continues to appear
your physician.
Movemen
Carefully read and
steps in section 3.3.
The batteries are low.
Y
with new ones ahead of time.
Refer to section 2.1.
The batteries are ex
Y
with new ones at once.
Refer to section 2.1.
Air plug disconnected.
Insert the p
Refer to section 3.1.
Arm cuff is applied
Apply the a
Refer to section 3.1.
Air is leaking from the arm cuf
Replace the cuff with a new one.
Refer to section 5.3.
Movemen
and the arm cuff has not
inflated suffi
Repeat measurement. Remain
still and do not
measurement.
Refer to section 3.3.
If “E2” appear
inflate the cuff manually until it is
30 to 40 m
previous measur
Refer to section 3.3.
The arm cuff was inflated above
299 mmHg wh
cuff manually
Do not inflate the cuff
mmHg
Refer to section 3.3.
Movemen
Repeat measurement. Remain
still and do not
measurement.
Refer to section 3.3.
Clothing is interfering with
arm cuff.
Remove any clothing interfering
with the arm
Refer to section 3.1.
Device error
Contact your OMRON r
outlet or distributor
Proble Cause Soluti
The measurement
result is extremely
high (or low).
Arm cuff is applied t
loosely
Apply the arm cuff tighter
Refer to section 3.1.
Movement or talking during
measurement.
Remain still and do not
during measurement.
Refer to section 3.3.
Clothing is interfering wi
the arm
Remove any clothing
interfering with the arm
Refer to section 3.1.
Arm cuff pressure
does not rise.
The air con
securely connected into the
air jack.
Make sure that the air tube
is connected securely
Refer to section 3.1.
Air is leaking from
cuf
Replace the arm
new one.
Refer to section 5.3.
The cuff wrapping
guide lamp does not
l
ight.
Arm cuff deflates too
soon.
The arm cuff is loose.
Apply the
that it is firmly wrapped
around the arm.
Refer to section 3.1.
Cannot measure or
the results are
or too high.
The arm cuff has not been
inflated suf
Inflate the cuff so that it is 3
to 40 mmHg above your
previous measurement
result.
Refer to section 3.3.
Nothing happens
when you press
buttons.
The batteries are empty
Replace the batteries w
new ones.
Refer to section 2.1.
The batteries have been
inserted incorrectly
Insert the batteries with the
correct (
Refer to section 2.1.
Other problems.
• Press the ST
measurement.
• Replace the batteries with new ones.
If the problem continues, contact your OMRON r
or distributor
Arm cuff AC adapter
Arm circumf
22 -
Easy Cuff L
991
(Model: HEM-RML
Adapter S
9515336-9
Adapter UK
9983666-5
Product description Automatic Blood Pres
Mode OMRON M3 (HEM-71
Display LCD Digit
Measurement
method
Oscillometric method
Measurement range Pressure: 0 t
Pulse: 40 to 18
Accuracy Pressure: ±3 mm
Pulse: ±5% of
Infla Fuzzy-logic controlled by electric pump
Deflation Automatic press
Memory 60 measurements with date and time for each us
Rating DC6V 4W
Power sour 4 “AA” batteries 1.5V or optional AC adapter
(Adapter S-9515336-9, I
(Adapter UK
9983666-5
, INPUT A
Battery life Approx. 1000
Applied part
= T
Protection against
electric shock
Internally powered ME equipment (When using only the
batteries)
= Class II ME equipment (Optional AC adapter)
Operating
temperature/
humi
+10 to +40°C / 30 to 85% RH
Storage
temperature/
humi
air pressure
-20 to +60°C /
IP classification IP 20
We Monitor: Approx. 280
Arm cuff: Approx. 170 g
Outer dimensions Monitor: A
Arm cuff: Approx. 145 mm x 594 mm
Cuff circumference 22 to 42 cm
Cuff/ T Nylon, poly
Package contents Monitor,
battery set, blood
Important information regarding Electro Magn
With the increased number of electronic devices such as PC’s and mobile (cellular)
telephones, medical devices
interference from other devices. Electromagnetic interference may result in
incorrect operation of the medical devi
Medical devices should also not interfere with other
In order to regulate the requirements for EMC (Electro Magnetic Compatibility) with
the aim to prevent
been implemented. This standard def
electromagnetic interferences as
emissions for medical devices.
This medical device m OMRON HEAL
EN60601-1-2:2007 standard fo
Nevertheless, special pr
• Do n
electrical or electromagnetic fields, near the medical device. This may result in
incorrect operation of the device and create a pot
Recommendation is to keep a minimum distance of 7 m. V
of the d
Further documentation in accordance with EN60601-1-2:2007 is available
OMRON HEAL
manual.
Documentation is also available at www.omron-healthcare.com
.
Correct Disposal of This Product
(W
This marking shown
should not be dispos
of its working life. T
human health from uncontrolled waste disposal, please sep
this product from other types of wastes and rec
promote the sustainable reuse of material resources.
Household users should contact either the ret
product, or their local government of
return this item
Business users should contact their supplier and check the term
the purchase contrac
wastes for disposal.
Manufacturer
OMRON HEAL
53, Kun
617-000
EU-representative
OMRON HEAL
Scorpius
www.omron-healt
Prod
OMRON HEAL
Binh Duo
Subs
OMRON HEAL
Opal Drive, Fo
OMRON MEDIZINTECHNIK HANDEL
Gottlieb-Da
www.omron-heal
OMRON SANTÉ FRANCE SAS
14, rue