A
Specification
T
W
Cleaning
Maintenanc
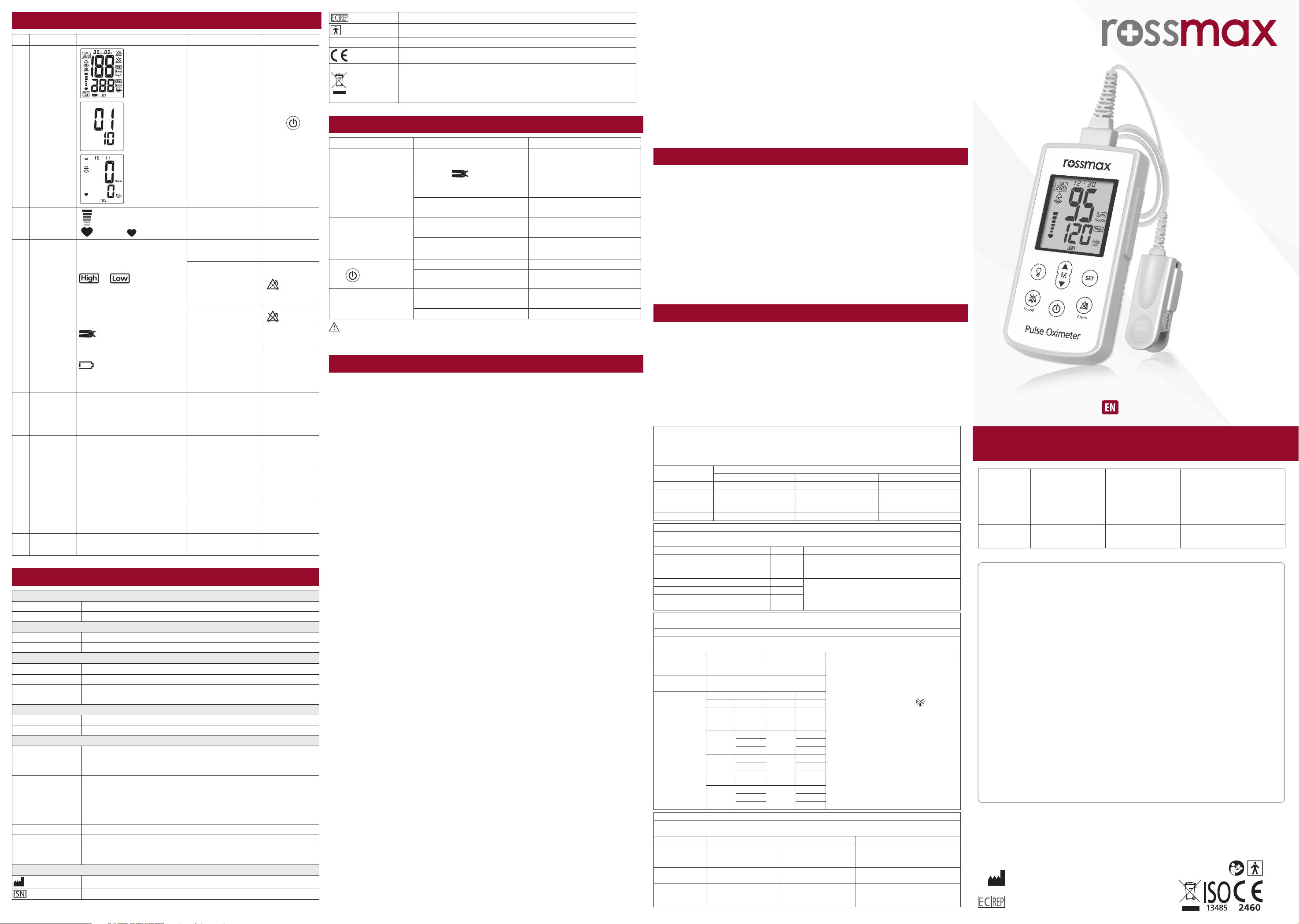
No Name L Sound Control
1P
All symbols
software
version
star
Beep for 2 second Pr for
1 second
2Pulse
search The ickers
X X
3
Max./ M
SpO2 and
Pulse rat
warning
or and yellow
backlight ick
Beep-beep
sounded repeatedly Default
Mute for t
will beep-beep
sounded after two
minutes.
mode
Mute befor
o mode
4Pr
failure alert
shows on the screen
and yellow back light icker
Beep-beep
sounded repeatedly X
5
Low
batter
aler
shows on the screen
and yellow backlight ickers.
Beep-beep
sounded repeatedly
for 1 minute and
power o/
x
6Automatic
O X
Beep-beep
sounded and then
power o
After pulse is
undetectable
for ar
minutes.
7Unable
measure
Blood saturation & pulse
rate appears (- -) and yellow
backlight ick
Beep- beep
sounded
repeatedly
X
8
Data
Updated
P
Less than 2 seconds X X
9
Aler
Condition
Delay
Less than 8 seconds f
SpO2. Less than 16 seconds
for pulse rate
X X
10 Aler
Delay
Less than 1 second f
and pulse rate X X
SpO2
Measuring range 35% – 100% (the resolution is 1%)
Accuracy 70% – 100%: ±2 %, 35% – 69%: unspecied
Pulse Rate
Measuring range 30 – 250 bpm (the resolution is 1 bpm)
Accuracy 30 – 250 ± 3 digits
Probe T
Pr Rossmax P
Extension cord Rossmax PE100
Optical Sensor The wavelength of r
905/880 nm with maximum optical output power of 4 mW/sr
Electric
Batter AA * 4 (Alkaline)
Batter Continually for 15 hours with 4 alkaline batteries
Envir
Operation
Condition
T
– 95%(non condensing), Atmospheric pressure: 700hP
1060hP
Storage /
T
Condition
T
Humidity
700hP
Note:
for 3 hours at r
Dimension Size: 14.5(L) x 7.25(
W About 150g (without the batteries)
Standard IEC/EN60601-1, IEC/EN60601-1-2, IEC/EN60601-1-11,
ISO80601-2-61
Symbol Descriptors
Manufacturer
Serial number
Symptoms Check points Corrections
SpO2 or Pulse rat
cannot displayed
The icon
screen
Place the nger properly
and tr
This icon shows on
the screen means probe
dysfunction
Be sure
connected to the device
correctly
Applied nger improperly Place the nger properly
and tr
SpO2 or Pulse rat
are not display
stably
F
moving Keep body steady
Applied nger improperly Place the nger properly
and tr
No display when
the bottom is
pressed
Batteries run down Replace with new batteries
Batteries inser Re
The display
disappears suddenly
The device will auto power
o when it gets no signal Normal
Low batt Replace with new batteries
No
shou
•
sen
su
from t
t
•
•
hemogl
the meas
-
or in
-
-
-
-
-
-
-
-
-
-
-
-
-
-
ac
can result
• the de for long per m cau pai for people with c disor
Repos
and to ch
•
•
an
•
•
•
th
•
pro
re
•
•
•
you
dosage of any existi
1
alc
fabr
2.
in
3.
not high
Not
an
ammoni
Reco
checks eve
• I
• E
Not
equipm
EMC guidance and manufacturer’s declaration
Recommended separation distanc
The Handheld P-
tomer or the user of the Handheld Pulse O
por
maximum output power of the communic
Rated maximum output
power of transmitt Separation distance ac
150 kHz to 80 MHz , d=[3.5/V1]√P 80 MHz to 800 MHz , d=[3.5/E1]√P 800 MHz to 2,5 GHz , d=[7/E1]√P
0.01 0.1 0.1 0.2
0.1 0.4 0.4 0.7
11.2 1.2 2.3
10 3.7 3.7 7.4
100 11.7 11.7 23.3
Declaration – electromagnetic emissions
The Handheld P-
held Pulse Oximet
Emissions test Complianc Elec
RF emissions CISPR 11 Group 1 T-
tion.
any interferenc
RF emissions CISPR 11 Class B The Handheld P
including domestic establishments and those dir
public low-voltage po-
plies buildings used for domestic purposes.
Harmonic emissions IEC 61000-3-2 N/A
V N/A
Declaration – electromagnetic emissions and immunity – for EQUIPMENT and SYSTEMS that ar
envir
The Handheld P
The Handheld P
Handheld Pulse Oximet
Immunity test IEC 60601 test level Compliance level Electromagnetic en
Conducted RF IEC
61000-4-6 3
to 80 MHz N/A Portable and mobile RF communications equipmen
be used no closer to any part of the EQUIPMENT or SYSTEM
including cables, than the r
calculated fr
the transmitter-
ment marked with the follo
Radiated RF IEC
61000-4-3 3
– 2.7 GHz l 80% 3
– 2.7 GHz ; 80%
Pr
RF wireless C-
cations equipment IEC
61000-4-3
27 V/m 385 MHz 27 V/m 385 MHz
28 V/m 450 MHz 28 V/m 450 MHz
9 V/m 710 MHz 9 V/m 710 MHz
745 MHz 745 MHz
780 MHz 780 MHz
28 V/m 810 MHz 28 V/m 810 MHz
870 MHz 870 MHz
930 MHz 930 MHz
28 V/m 1720 MHz 28 V/m 1720 MHz
1845 MHz 1845 MHz
1970 MHz 1970 MHz
28 V/m 2450 MHz 28 V/m 2450 MHz
9 V/m 5240 MHz 9 V/m 5240 MHz
5500 MHz 5500 MHz
5785 MHz 5785 MHz
Declaration – electromagnetic immunity
The Handheld P
Handheld Pulse Oximet
Immunity test IEC 60601 test level C Electromagnetic en
Electrosta
(ESD) IEC 61000-4-2 ±8 kV c
±2 kV ±8 kV c
±2 kV F
If oors are c
rela
Electrical fast transient/
burst IEC 61000-4-4 ±2 kV for power supply lines
±1 kV for input/output lines N/A Mains power quality should be that of a typical
commer
Surge IEC 61000-4-5 ±0.5 kV
±1 kV dieren
±2 kV common mode
N/A Mains power quality should be that of a typical
commer
EU representativ
T
IP Classication IP22: Pr
CE Mark
W
electronic product and following the E
2012/19/EU the electronic products have to be dispose on
your local recycling centre f
• no look di ins the housi du the me T red l and the
in
•
•
pat
• A wa
• Do no
•
c
•
life
V
interruptions and
voltage varia
power supply input
lines IEC 61000-4-11
0 % UT; 0, 5 c
135°, 180°, 225°, 270° and 315°
0 % UT; 1 cT;
25/30 cycle Single phase: at 0°
N/A Mains power quality should be that of a typical
commer
of the EQUIPMENT or SYSTEM r-
ued operation during pow-
tions, it is r
or SYSTEM be pow
power supply or a ba
Po
(50/60 Hz) magnetic
eld IEC 61000-4-8
30 A/m 30 A/m Po
at levels char
typical commercial or hospital en
RI_IB_SA120_EN_
TP_ver2021
Warranty Card
This instr
accessor
car
the instrument in
non-
r
Customer Name: _________________________________________
Address: ______________________________________________
T ____________________________________________
E-mail address: __________________________________________
Product Information:
Date of purchase: ________________________________________
Store where purchased:
_____________________________________________________
Model: SA120
w
IN0SA120000000060
Rossmax Inno
12F
CMC Medical Devices & Drugs S.L.
C/ Horacio L
Handheld Pulse Oximeter
* The text is s